Unlock Scalable, High-Yield Protein Expression with Pfenex Expression Technology®
The Pfenex Expression Technology® (Pfenex) is the most comprehensive prokaryotic expression platform under one roof: powered by Pseudomonas fluorescens.
About P. fluorescens
Advantages of Pfenex
Applying Pfenex

Pfast™ Feasibility Program
Success Stories
About P. fluorescens
Advantages of Pfenex
Applying Pfenex
Pfast™ Feasibility Program
Success Stories
ABOUT Pseudomonas fluorescens
A naturally occurring, Gram-negative obligate aerobe, our P. fluorescens was first isolated from a lettuce leaf around San Diego, California. Initially developed by The Dow Chemical Company with the intent of being a robust, scalable, and cost-focused expression technology from the start, Primrose’s P. fluorescens-based Pfenex platform is unmatched in recombinant protein production:
- 100% compatible with existing microbial know-how, production systems, and infrastructure.
- Used for producing industrial products and human therapeutics since the 1990s.
- Large genome (6.3 Mbp) provides a greater metabolic diversity over E. coli (5 Mbp average).
- High density culture conditions (30-50 OD) are achieved at 0.5mL scale, and has successfully been scaled >180,000 liters (200-400 OD).
- Cultivates with simple, chemically defined and animal origin-free media. No antibiotics are required for plasmid maintenance.
We leverage these benefits of P. fluorescens to optimize protein expression, from initial DNA transcription through final protein folding and secretion.
PFENEX EXPRESSION TECHNOLOGY ADVANTAGES
Unmatched Versatility and Performance:
The Pfenex platform combines decades of dedicated development with proven success in the production of complex, challenging proteins.
Pfenex succeeds where E. coli and CHO often fall short.
The Pfenex Toolbox
25+ years of engineering toolbox components, including highly efficient secretion leaders, plasmids, promoters, ribosome binding sites, and chaperones.

High throughput
Compatible with automated strain engineering tools for rapid, efficient screening of thousands of gene-vector-strain combinations at once.
High titers, high quality
Historically improves expression titer and quality with an ~80% success rate, notably with complex structures (e.g., disulfide bonds).
Scalable
Up to 180,000L achieved for industrial products; 5,000L for therapeutics.
Cost-effective
Titers exceeding 30g/L have been achieved; periplasmic release offers cleaner downstream processing.

Versatile
Over 200 lead candidates produced from a variety of protein modalities: VHHs, Fabs, and other antibody derivatives; enzymes, cytokines, antigens, and peptides.




How it Works
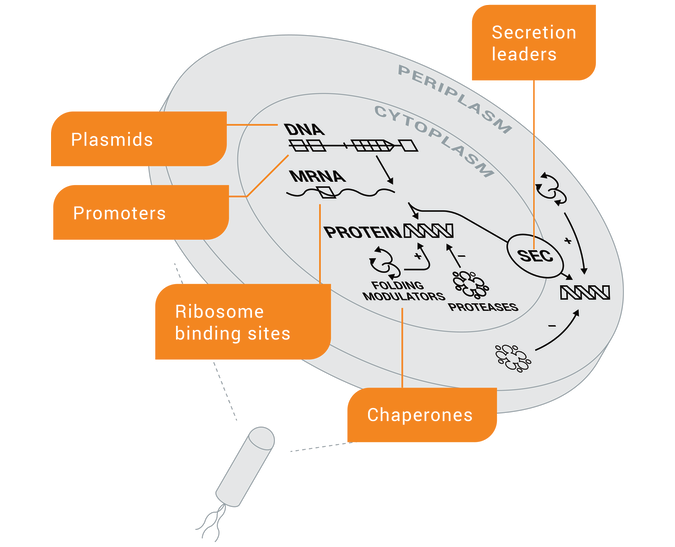
Secretion Leaders
Plasmids
Ribosome Binding Sites
Chaperone Proteins
Host-strain Protease Mutants
Working with Primrose Bio
Transform your protein expression challenges into solutions with Pfenex. Our streamlined strain engineering process consistently delivers actionable results for the most challenging of proteins while maintaining rigorous quality standards. It seamelessly integrates with your existing microbial production capabilities.
Initial Consultation
Proposal Development
Advanced Strain Engineering
Primary Process Optimization
Cell Banking
Licensing


Pfast™ Feasibility with Pfenex
Accelerate Development with the Pfast Protein Feasibility Assessment
The Pfast™ Feasibility Program offers a rapid, cost-effective way to assess your molecule's compatibility with the Pfenex platform. Designed for early-stage evaluation, Pfast enables you to make data-driven decisions before committing to full-scale development.
Proven Success Across Global Markets
Serum Institute of India: Pneumosil® and Men-Five® use Pfenex-produced CRM197 as their carrier protein and have been administered to over 45 million children, worldwide.
Alvogen: A biosimilar to Eli Lilly’s Forteo®, Teriparatide Injection for the treatment of post-menopausal osteoporosis was the first approved drug produced using the Pfenex platform.
Merck & Co., Inc.: CRM197 produced via the Pfenex platform is used in two FDA and EMA approved pneumococcal vaccines, VAXNEUVANCE® and CAPVAXIVE®.
Jazz Pharmaceuticals: Rylaze® achieved FDA approval in under six years from initial DNA cloning, demonstrating Pfenex’s speed and efficiency.

All trademarks here not owned by Primrose are owned by their respective holders.
